Titration Calculations Acid Base . To evaluate the relationship between a titration’s equivalence point and its end point we need to. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. In an indicator based titration you add another. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Updated on november 26, 2019. There are two basic types of acid base titrations, indicator and potentiometric. Moles acid = moles base moles.
from www.chegg.com
In an indicator based titration you add another. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. To evaluate the relationship between a titration’s equivalence point and its end point we need to. Updated on november 26, 2019. Moles acid = moles base moles. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. There are two basic types of acid base titrations, indicator and potentiometric.
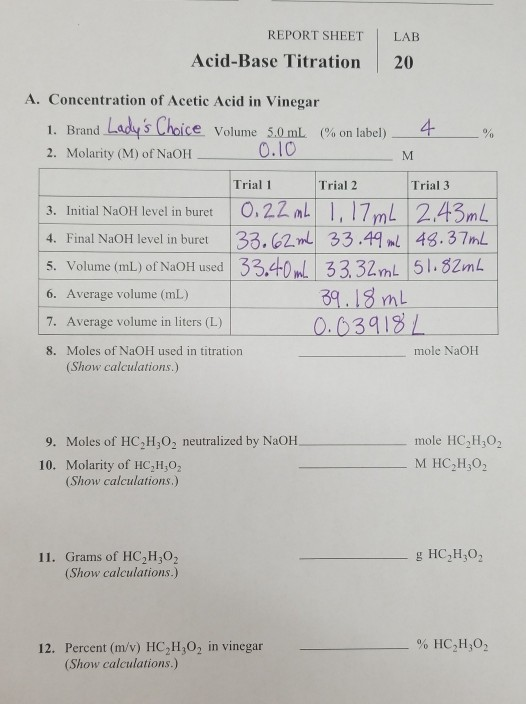
Solved REPORT SHEET LAB AcidBase Titration 20 A.
Titration Calculations Acid Base There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Updated on november 26, 2019. Moles acid = moles base moles. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. To evaluate the relationship between a titration’s equivalence point and its end point we need to.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Acid Base Moles acid = moles base moles. Updated on november 26, 2019. In an indicator based titration you add another. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. At the equivalence point in a neutralization, the moles of acid are equal to the moles. Titration Calculations Acid Base.
From www.youtube.com
Calculations for Titration of Weak Base with Strong Acid, initial pH Titration Calculations Acid Base Updated on november 26, 2019. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m. Titration Calculations Acid Base.
From mungfali.com
Acid Base Titration Procedure Titration Calculations Acid Base In an indicator based titration you add another. Updated on november 26, 2019. To evaluate the relationship between a titration’s equivalence point and its end point we need to. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Moles acid = moles base moles. There are two basic types of acid. Titration Calculations Acid Base.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Acid Base There are two basic types of acid base titrations, indicator and potentiometric. Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. Updated on november 26, 2019. To evaluate the relationship between a titration’s equivalence point and its end. Titration Calculations Acid Base.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Acid Base Updated on november 26, 2019. In an indicator based titration you add another. To evaluate the relationship between a titration’s equivalence point and its end point we need to. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Moles acid = moles base moles. There are two basic types of acid. Titration Calculations Acid Base.
From www.youtube.com
Worked example Determining solute concentration by acidbase titration Titration Calculations Acid Base To evaluate the relationship between a titration’s equivalence point and its end point we need to. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. Updated on november 26, 2019. Moles acid = moles base moles. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Acid Base.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Titration Calculations Acid Base To evaluate the relationship between a titration’s equivalence point and its end point we need to. Updated on november 26, 2019. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and. Titration Calculations Acid Base.
From mavink.com
Acid Base Titration Equation Titration Calculations Acid Base Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. There are two basic types of acid base titrations, indicator and potentiometric. To evaluate the relationship between a titration’s equivalence point and its end point we need to. Updated. Titration Calculations Acid Base.
From www.thoughtco.com
AcidBase Titration Calculation Titration Calculations Acid Base To evaluate the relationship between a titration’s equivalence point and its end point we need to. In an indicator based titration you add another. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. Moles acid = moles base moles. At the equivalence point in. Titration Calculations Acid Base.
From www.youtube.com
Acid Base Titration Calculation Example YouTube Titration Calculations Acid Base In an indicator based titration you add another. Updated on november 26, 2019. To evaluate the relationship between a titration’s equivalence point and its end point we need to. There are two basic types of acid base titrations, indicator and potentiometric. Moles acid = moles base moles. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Acid Base.
From mungfali.com
Acid Base Titration Formula Titration Calculations Acid Base Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. To evaluate the relationship between a titration’s equivalence point and its end point we need to. In. Titration Calculations Acid Base.
From mungfali.com
Acid Base Titration Calculation Titration Calculations Acid Base To evaluate the relationship between a titration’s equivalence point and its end point we need to. Updated on november 26, 2019. In an indicator based titration you add another. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Calculate the ph for the weak acid/strong base titration between 50.0 ml of. Titration Calculations Acid Base.
From pressbooks.online.ucf.edu
15.7 AcidBase Titrations Chemistry Fundamentals Titration Calculations Acid Base Updated on november 26, 2019. In an indicator based titration you add another. There are two basic types of acid base titrations, indicator and potentiometric. Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. At the equivalence point. Titration Calculations Acid Base.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Calculations Acid Base In an indicator based titration you add another. Moles acid = moles base moles. There are two basic types of acid base titrations, indicator and potentiometric. Updated on november 26, 2019. To evaluate the relationship between a titration’s equivalence point and its end point we need to. At the equivalence point in a neutralization, the moles of acid are equal. Titration Calculations Acid Base.
From www.studocu.com
Chapter 14 Acid Base Titration Calculations in the context of Titration Calculations Acid Base Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. Updated on november 26, 2019. There are two basic types of acid base titrations, indicator and potentiometric. To evaluate the relationship between a titration’s equivalence point and its end. Titration Calculations Acid Base.
From www.sliderbase.com
Titration Titration Calculations Acid Base Moles acid = moles base moles. At the equivalence point in a neutralization, the moles of acid are equal to the moles of base. Updated on november 26, 2019. To evaluate the relationship between a titration’s equivalence point and its end point we need to. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator. Titration Calculations Acid Base.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Calculations Acid Base Updated on november 26, 2019. Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. There are two basic types of acid base titrations, indicator and potentiometric. In an indicator based titration you add another. At the equivalence point. Titration Calculations Acid Base.
From www.slideserve.com
PPT Unit 19 Acid Base Equilibria Titrations PowerPoint Presentation Titration Calculations Acid Base Moles acid = moles base moles. Calculate the ph for the weak acid/strong base titration between 50.0 ml of 0.100 m hcooh(aq) (formic acid) and 0.200 m naoh (titrant) at the. There are two basic types of acid base titrations, indicator and potentiometric. To evaluate the relationship between a titration’s equivalence point and its end point we need to. At. Titration Calculations Acid Base.